what is a branched polymer? cite your source and be sure to define this term in your own words.
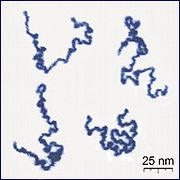
Appearance of real linear polymer chains as recorded using an diminutive force microscope on a surface, under liquid medium. Chain contour length for this polymer is ~204 nm; thickness is ~0.iv nm.[1]
A polymer is a substance composed of macromolecules.[2] A macromolecule is a molecule of high relative molecular mass, the structure of which substantially comprises the multiple repetition of units derived, really or conceptually, from molecules of low relative molecular mass.[3]
A polymer (;[4] [v] Greek poly-, "many" + -mer, "part") is a substance or fabric consisting of very large molecules, or macromolecules, composed of many repeating subunits.[six] Due to their broad spectrum of backdrop,[7] both constructed and natural polymers play essential and ubiquitous roles in everyday life.[8] Polymers range from familiar constructed plastics such as polystyrene to natural biopolymers such as Deoxyribonucleic acid and proteins that are key to biological structure and office. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known every bit monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.
The term "polymer" derives from the Greek word πολύς (polus, pregnant "many, much") and μέρος (meros, meaning "part"). The term was coined in 1833 past Jöns Jacob Berzelius, though with a definition distinct from the modernistic IUPAC definition.[9] [ten] The modern concept of polymers as covalently bonded macromolecular structures was proposed in 1920 past Hermann Staudinger,[11] who spent the next decade finding experimental evidence for this hypothesis.[12]
Polymers are studied in the fields of polymer scientific discipline (which includes polymer chemistry and polymer physics), biophysics and materials science and engineering. Historically, products arising from the linkage of repeating units by covalent chemical bonds have been the primary focus of polymer scientific discipline. An emerging important area now focuses on supramolecular polymers formed by non-covalent links. Polyisoprene of latex rubber is an example of a natural polymer, and the polystyrene of styrofoam is an example of a synthetic polymer. In biological contexts, substantially all biological macromolecules—i.e., proteins (polyamides), nucleic acids (polynucleotides), and polysaccharides—are purely polymeric, or are composed in large part of polymeric components.

Cartoon schematic of polymer molecules
Common examples
Polymers are of two types: naturally occurring and constructed or man made.
Natural
Natural polymeric materials such as hemp, shellac, amber, wool, silk, and natural rubber have been used for centuries. A variety of other natural polymers be, such as cellulose, which is the main constituent of wood and newspaper.
Synthetic
The listing of synthetic polymers, roughly in order of worldwide need, includes polyethylene, polypropylene, polystyrene, polyvinyl chloride, constructed rubber, phenol formaldehyde resin (or Bakelite), neoprene, nylon, polyacrylonitrile, PVB, silicone, and many more. More than 330 one thousand thousand tons of these polymers are made every year (2015).[13]
Most commonly, the continuously linked courage of a polymer used for the grooming of plastics consists mainly of carbon atoms. A simple example is polyethylene ('polythene' in British English), whose repeat unit or monomer is ethylene. Many other structures do exist; for case, elements such equally silicon form familiar materials such as silicones, examples being Featherbrained Putty and waterproof plumbing sealant. Oxygen is as well commonly nowadays in polymer backbones, such equally those of polyethylene glycol, polysaccharides (in glycosidic bonds), and DNA (in phosphodiester bonds).
History
Polymers accept been essential components of commodities since the early days of humankind. The use of wool (keratin), cotton wool and linen fibres (cellulose) for garments, newspaper reed (cellulose) for newspaper are just a few examples of how our ancestors exploited polymer-containing raw materials to obtain artefacts. The latex sap of "caoutchouc" trees (natural rubber) reached Europe in the 16th century from Southward America long after the Olmec, Maya and Aztec had started using it every bit a fabric to brand balls, waterproof textiles and containers.[14]
The chemical manipulation of polymers dates back to the 19th century, although at the fourth dimension the nature of these species was not understood. The behaviour of polymers was initially rationalised according to the theory proposed by Thomas Graham which considered them as colloidal aggregates of small molecules held together by unknown forces.
Notwithstanding the lack of theoretical knowledge, the potential of polymers to provide innovative, accessible and cheap materials was immediately grasped. The work carried out by Braconnot, Parkes, Ludersdorf, Hayard and many others on the modification of natural polymers determined many meaning advances in the field.[15] Their contributions led to the discovery of materials such as celluloid, galalith, parkesine, rayon, vulcanised rubber and, later, Bakelite: all materials that quickly entered industrial manufacturing processes and reached households as garments components (e.thousand., fabrics, buttons), crockery and decorative items.
In 1920, Hermann Staudinger published his seminal work "Über Polymerisation",[16] in which he proposed that polymers were in fact long chains of atoms linked past covalent bonds. His piece of work was debated at length, simply eventually it was accepted by the scientific community. Considering of this piece of work, Staudinger was awarded the Nobel Prize in 1953.[17]
After the 1930s polymers entered a gold age during which new types were discovered and quickly given commercial applications, replacing naturally-sourced materials. This development was fuelled past an industrial sector with a strong economic drive and information technology was supported by a wide academic community that contributed with innovative synthesis of monomers from cheaper raw materials, more efficient polymerisation processes, improved techniques for polymer characterisation and advanced theoretical understanding of polymers.[15]

Since 1953, six Nobel prizes were awarded in the area of polymer science, excluding those for inquiry on biological macromolecules. This further testifies its touch on on modern science and technology. As Lord Todd summarised information technology in 1980, "I am inclined to recall that the evolution of polymerization is perhaps the biggest affair that chemistry has done, where it has had the biggest effect on everyday life".[19]
Synthesis
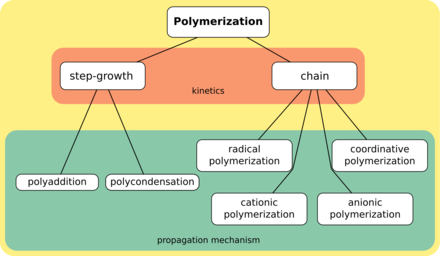
A classification of the polymerization reactions
Polymerization is the process of combining many minor molecules known as monomers into a covalently bonded chain or network. During the polymerization process, some chemical groups may exist lost from each monomer. This happens in the polymerization of PET polyester. The monomers are terephthalic acrid (HOOC—C6H4—COOH) and ethylene glycol (HO—CH2—CHii—OH) only the repeating unit is —OC—C6Hiv—COO—CHii—CH2—O—, which corresponds to the combination of the two monomers with the loss of 2 water molecules. The distinct piece of each monomer that is incorporated into the polymer is known every bit a repeat unit of measurement or monomer remainder.
Constructed methods are generally divided into two categories, step-growth polymerization and chain polymerization.[twenty] The essential divergence between the 2 is that in chain polymerization, monomers are added to the chain ane at a time only,[21] such as in polystyrene, whereas in step-growth polymerization chains of monomers may combine with 1 another straight,[22] such every bit in polyester. Step-growth polymerization tin be divided into polycondensation, in which low-molar-mass by-product is formed in every reaction stride, and polyaddition.

Example of chain polymerization: Radical polymerization of styrene, R. is initiating radical, P. is some other polymer chain radical terminating the formed chain by radical recombination
Newer methods, such equally plasma polymerization exercise not fit neatly into either category. Synthetic polymerization reactions may be carried out with or without a goad. Laboratory synthesis of biopolymers, especially of proteins, is an area of intensive inquiry.
Biological synthesis

There are three main classes of biopolymers: polysaccharides, polypeptides, and polynucleotides. In living cells, they may be synthesized by enzyme-mediated processes, such as the formation of Deoxyribonucleic acid catalyzed by Dna polymerase. The synthesis of proteins involves multiple enzyme-mediated processes to transcribe genetic information from the Dna to RNA and afterwards interpret that information to synthesize the specified protein from amino acids. The protein may exist modified further following translation in order to provide appropriate structure and functioning. There are other biopolymers such every bit rubber, suberin, melanin, and lignin.
Modification of natural polymers
Naturally occurring polymers such as cotton fiber, starch, and rubber were familiar materials for years earlier synthetic polymers such as polyethene and perspex appeared on the market. Many commercially of import polymers are synthesized past chemical modification of naturally occurring polymers. Prominent examples include the reaction of nitric acid and cellulose to form nitrocellulose and the formation of vulcanized rubber past heating natural rubber in the presence of sulfur. Ways in which polymers tin can be modified include oxidation, cross-linking, and endcapping.
Structure
The structure of a polymeric material tin can exist described at different length scales, from the sub-nm length scale up to the macroscopic ane. At that place is in fact a hierarchy of structures, in which each stage provides the foundations for the next one.[23] The starting point for the description of the structure of a polymer is the identity of its constituent monomers. Next, the microstructure essentially describes the organisation of these monomers within the polymer at the calibration of a single chain. The microstructure determines the possibility for the polymer to form phases with different arrangements, for example through crystallization, the glass transition or microphase separation.[24] These features play a major part in determining the physical and chemic properties of a polymer.
Monomers and repeat units
The identity of the repeat units (monomer residues, also known as "mers") comprising a polymer is its get-go and nigh important attribute. Polymer nomenclature is by and large based upon the type of monomer residues comprising the polymer. A polymer which contains only a single blazon of echo unit is known as a homopolymer, while a polymer containing two or more types of repeat units is known as a copolymer.[25] A terpolymer is a copolymer which contains 3 types of echo units.[26]
Polystyrene is composed simply of styrene-based echo units, and is classified as a homopolymer. Polyethylene terephthalate, even though produced from two different monomers (ethylene glycol and terephthalic acrid), is usually regarded as a homopolymer because but one type of repeat unit is formed. Ethylene-vinyl acetate contains more than i variety of echo unit and is a copolymer. Some biological polymers are composed of a variety of different but structurally related monomer residues; for case, polynucleotides such as DNA are composed of four types of nucleotide subunits.
-
Homopolymers and copolymers (examples) 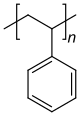
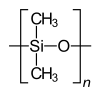
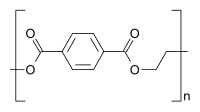
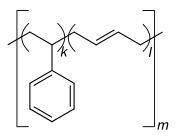
Homopolymer polystyrene Homopolymer polydimethylsiloxane, a silicone. The main chain is formed of silicon and oxygen atoms. The homopolymer polyethylene terephthalate has only 1 repeat unit. Copolymer styrene-butadiene safety: The echo units based on styrene and 1,3-butadiene class two repeating units, which can alternate in any order in the macromolecule, making the polymer thus a random copolymer.
A polymer containing ionizable subunits (e.g., pendant carboxylic groups) is known as a polyelectrolyte or ionomer, when the fraction of ionizable units is large or pocket-sized respectively.
Microstructure
The microstructure of a polymer (sometimes called configuration) relates to the physical arrangement of monomer residues along the backbone of the chain.[27] These are the elements of polymer structure that require the breaking of a covalent bond in guild to change. Various polymer structures can be produced depending on the monomers and reaction atmospheric condition: A polymer may consist of linear macromolecules containing each only ane unbranched concatenation. In the case of unbranched polyethylene, this chain is a long-concatenation n-alkane. There are also branched macromolecules with a master chain and side chains, in the example of polyethylene the side chains would be alkyl groups. In particular unbranched macromolecules can be in the solid state semi-crystalline, crystalline chain sections highlighted ruddy in the effigy below.
While branched and unbranched polymers are usually thermoplastics, many elastomers have a wide-meshed cantankerous-linking between the "main bondage". Close-meshed crosslinking, on the other mitt, leads to thermosets. Cantankerous-links and branches are shown as red dots in the figures. Highly branched polymers are amorphous and the molecules in the solid interact randomly.
-

linear, unbranched macromolecule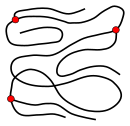
branched macromolecule
semi-crystalline structure of an unbranched polymer
slightly cross-linked polymer (elastomer)
highly cross-linked polymer (thermoset)
Polymer architecture
![]()
Co-operative signal in a polymer
An of import microstructural feature of a polymer is its architecture and shape, which relates to the way branch points pb to a deviation from a uncomplicated linear concatenation.[28] A branched polymer molecule is equanimous of a master chain with one or more substituent side chains or branches. Types of branched polymers include star polymers, comb polymers, polymer brushes, dendronized polymers, ladder polymers, and dendrimers.[28] At that place exist also two-dimensional polymers (2DP) which are composed of topologically planar repeat units. A polymer's architecture affects many of its physical properties including solution viscosity, melt viscosity, solubility in diverse solvents, glass-transition temperature and the size of individual polymer coils in solution. A diverseness of techniques may exist employed for the synthesis of a polymeric material with a range of architectures, for example living polymerization.
Chain length
A common means of expressing the length of a chain is the degree of polymerization, which quantifies the number of monomers incorporated into the concatenation.[29] [xxx] As with other molecules, a polymer's size may also be expressed in terms of molecular weight. Since synthetic polymerization techniques typically yield a statistical distribution of chain lengths, the molecular weight is expressed in terms of weighted averages. The number-boilerplate molecular weight (Grand due north) and weight-boilerplate molecular weight (1000 w) are most commonly reported.[31] [32] The ratio of these ii values (M w / Thousand n) is the dispersity (Đ), which is commonly used to express the width of the molecular weight distribution.[33]
The physical properties[34] of polymer strongly depend on the length (or equivalently, the molecular weight) of the polymer chain.[35] Ane important example of the physical consequences of the molecular weight is the scaling of the viscosity (resistance to flow) in the melt.[36] The influence of the weight-average molecular weight ( ) on the melt viscosity ( ) depends on whether the polymer is above or below the onset of entanglements. Beneath the entanglement molecular weight[ description needed ], , whereas higher up the entanglement molecular weight, . In the latter case, increasing the polymer concatenation length 10-fold would increase the viscosity over thou times.[37] [ folio needed ] Increasing concatenation length furthermore tends to decrease chain mobility, increase strength and toughness, and increase the glass-transition temperature (Tchiliad).[38] This is a event of the increase in chain interactions such as van der Waals attractions and entanglements that come with increased chain length.[39] [xl] These interactions tend to ready the individual chains more strongly in position and resist deformations and matrix breakup, both at higher stresses and higher temperatures.
Monomer organization in copolymers
Copolymers are classified either as statistical copolymers, alternating copolymers, block copolymers, graft copolymers or slope copolymers. In the schematic figure below, Ⓐ and Ⓑ symbolize the two repeat units.
- Alternate copolymers possess 2 regularly alternating monomer residues:[41] {{not a typo[AB]n}}. An example is the equimolar copolymer of styrene and maleic anhydride formed by costless-radical chain-growth polymerization.[42] A stride-growth copolymer such every bit Nylon 66 can as well exist considered a strictly alternating copolymer of diamine and diacid residues, just is often described as a homopolymer with the dimeric rest of ane amine and i acid as a echo unit.[43]
- Periodic copolymers have more than than two species of monomer units in a regular sequence.[44]
- Statistical copolymers accept monomer residues arranged co-ordinate to a statistical rule. A statistical copolymer in which the probability of finding a item type of monomer residuum at a item point in the chain is independent of the types of surrounding monomer residuum may be referred to as a truly random copolymer.[45] [46] For example, the chain-growth copolymer of vinyl chloride and vinyl acetate is random.[42]
- Block copolymers accept long sequences of different monomer units.[42] [43] Polymers with ii or three blocks of 2 distinct chemic species (east.g., A and B) are called diblock copolymers and triblock copolymers, respectively. Polymers with iii blocks, each of a different chemical species (e.g., A, B, and C) are termed triblock terpolymers.
- Graft or grafted copolymers comprise side chains or branches whose echo units have a different composition or configuration than the main chain.[43] The branches are added on to a preformed primary chain macromolecule.[42]
Monomers inside a copolymer may exist organized along the backbone in a multifariousness of ways. A copolymer containing a controlled organization of monomers is called a sequence-controlled polymer.[47] Alternating, periodic and block copolymers are elementary examples of sequence-controlled polymers.
Tacticity
Tacticity describes the relative stereochemistry of chiral centers in neighboring structural units within a macromolecule. There are 3 types of tacticity: isotactic (all substituents on the aforementioned side), atactic (random placement of substituents), and syndiotactic (alternating placement of substituents).
Morphology
Polymer morphology generally describes the arrangement and microscale ordering of polymer bondage in space. The macroscopic concrete properties of a polymer are related to the interactions between the polymer chains.
- Matted polymers: In the solid land, atactic polymers, polymers with a high caste of branching and random copolymers form amorphous (i.eastward. glassy structures).[48] In cook and solution, polymers tend to form a constantly changing "statistical cluster", encounter freely-jointed-concatenation model. In the solid land, the respective conformations of the molecules are frozen. Hooking and entanglement of chain molecules lead to a "mechanical bond" between the bondage. Intermolecular and intramolecular attractive forces only occur at sites where molecule segments are close plenty to each other. The irregular structures of the molecules preclude a narrower organization.
- Linear polymers with periodic structure, low branching and stereoregularity (e. chiliad. non atactic) take a semi-crystalline structure in the solid state.[48] In simple polymers (such as polyethylene), the chains are nowadays in the crystal in zigzag conformation. Several zigzag conformations course dense chain packs, called crystallites or lamellae. The lamellae are much thinner than the polymers are long (oft most 10 nm).[49] They are formed by more than or less regular folding of 1 or more molecular chains. Amorphous structures exist between the lamellae. Private molecules can lead to entanglements between the lamellae and can as well be involved in the germination of two (or more) lamellae (bondage than called necktie molecules). Several lamellae form a superstructure, a spherulite, oftentimes with a diameter in the range of 0.05 to i mm.[49]
- The type and organisation of (functional) residues of the repeat units effects or determines the crystallinity and strength of the secondary valence bonds. In isotactic polypropylene, the molecules form a helix. Like the zigzag conformation, such helices allow a dumbo chain packing. Particularly potent intermolecular interactions occur when the residues of the repeating units permit the formation of hydrogen bonds, as in the case of p-aramid. The formation of potent intramolecular associations may produce diverse folded states of unmarried linear chains with distinct circuit topology. Crystallinity and superstructure are always dependent on the weather of their formation, encounter also: crystallization of polymers. Compared to baggy structures, semi-crystalline structures lead to a college stiffness, density, melting temperature and higher resistance of a polymer.
- Cross-linked polymers: Wide-meshed cantankerous-linked polymers are elastomers and cannot exist molten (unlike thermoplastics); heating cross-linked polymers just leads to decomposition. Thermoplastic elastomers, on the other hand, are reversibly "physically crosslinked" and can exist molten. Block copolymers in which a hard segment of the polymer has a trend to crystallize and a soft segment has an amorphous structure are one type of thermoplastic elastomers: the difficult segments ensure wide-meshed, concrete crosslinking.
 wide-meshed cantankerous-linked polymer (elastomer) |  wide-meshed cantankerous-linked polymer (elastomer) under tensile stress |  crystallites as "crosslinking sites": one type of thermoplastic elastomer |  semi-crystalline thermoplastic elastomer under tensile stress |
Crystallinity
When applied to polymers, the term crystalline has a somewhat ambiguous usage. In some cases, the term crystalline finds identical usage to that used in conventional crystallography. For instance, the construction of a crystalline protein or polynucleotide, such as a sample prepared for x-ray crystallography, may be defined in terms of a conventional unit cell equanimous of one or more polymer molecules with cell dimensions of hundreds of angstroms or more. A constructed polymer may be loosely described as crystalline if it contains regions of three-dimensional ordering on diminutive (rather than macromolecular) length scales, usually arising from intramolecular folding or stacking of adjacent bondage. Synthetic polymers may consist of both crystalline and baggy regions; the caste of crystallinity may exist expressed in terms of a weight fraction or volume fraction of crystalline material. Few constructed polymers are entirely crystalline.[50] The crystallinity of polymers is characterized by their degree of crystallinity, ranging from zip for a completely non-crystalline polymer to one for a theoretical completely crystalline polymer. Polymers with microcrystalline regions are by and large tougher (tin can be bent more without breaking) and more bear on-resistant than totally amorphous polymers.[51] Polymers with a caste of crystallinity approaching zero or 1 will tend to exist transparent, while polymers with intermediate degrees of crystallinity will tend to be opaque due to light handful by crystalline or burnished regions. For many polymers, crystallinity may besides be associated with decreased transparency.
Chain conformation
The space occupied past a polymer molecule is generally expressed in terms of radius of gyration, which is an average altitude from the eye of mass of the chain to the concatenation itself. Alternatively, it may be expressed in terms of pervaded volume, which is the book spanned past the polymer chain and scales with the cube of the radius of gyration.[52] The simplest theoretical models for polymers in the molten, amorphous state are platonic chains.
Properties
Polymer backdrop depend of their structure and they are divided into classes co-ordinate to their physical basis. Many concrete and chemical properties describe how a polymer behaves every bit a continuous macroscopic material. They are classified as bulk backdrop, or intensive properties according to thermodynamics.
Mechanical properties

A polyethylene sample that has necked nether tension.
The majority properties of a polymer are those nigh frequently of cease-use interest. These are the properties that dictate how the polymer actually behaves on a macroscopic scale.
Tensile strength
The tensile force of a material quantifies how much elongating stress the fabric will endure before failure.[53] [54] This is very important in applications that rely upon a polymer's physical strength or durability. For case, a condom ring with a college tensile strength will hold a greater weight before snapping. In general, tensile strength increases with polymer chain length and crosslinking of polymer chains.
Immature's modulus of elasticity
Young's modulus quantifies the elasticity of the polymer. It is divers, for small strains, as the ratio of charge per unit of modify of stress to strain. Like tensile strength, this is highly relevant in polymer applications involving the physical properties of polymers, such equally rubber bands. The modulus is strongly dependent on temperature. Viscoelasticity describes a complex time-dependent elastic response, which will exhibit hysteresis in the stress-strain curve when the load is removed. Dynamic mechanical analysis or DMA measures this complex modulus by oscillating the load and measuring the resulting strain equally a function of time.
Transport backdrop
Send properties such as diffusivity depict how chop-chop molecules motility through the polymer matrix. These are very important in many applications of polymers for films and membranes.
The motility of individual macromolecules occurs by a process chosen reptation in which each concatenation molecule is constrained by entanglements with neighboring chains to move inside a virtual tube. The theory of reptation tin explain polymer molecule dynamics and viscoelasticity.[55]
Phase beliefs
Crystallization and melting
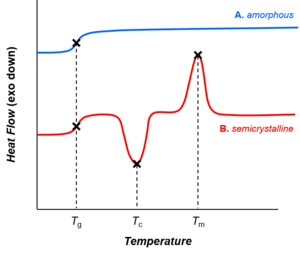
Thermal transitions in (A) amorphous and (B) semicrystalline polymers, represented equally traces from differential scanning calorimetry. As the temperature increases, both baggy and semicrystalline polymers get through the glass transition (T thou). Amorphous polymers (A) do non showroom other phase transitions, though semicrystalline polymers (B) undergo crystallization and melting (at temperatures T c and T m, respectively).
Depending on their chemical structures, polymers may exist either semi-crystalline or amorphous. Semi-crystalline polymers can undergo crystallization and melting transitions, whereas baggy polymers practice not. In polymers, crystallization and melting practice non propose solid-liquid phase transitions, every bit in the example of water or other molecular fluids. Instead, crystallization and melting refer to the phase transitions betwixt ii solid states (i.east., semi-crystalline and amorphous). Crystallization occurs to a higher place the glass-transition temperature (T thousand) and below the melting temperature (T m).
Drinking glass transition
All polymers (amorphous or semi-crystalline) go through drinking glass transitions. The drinking glass-transition temperature (T k) is a crucial physical parameter for polymer manufacturing, processing, and apply. Below T one thousand, molecular motions are frozen and polymers are brittle and glassy. In a higher place T g, molecular motions are activated and polymers are rubbery and viscous. The glass-transition temperature may be engineered by altering the caste of branching or crosslinking in the polymer or past the add-on of plasticizers.[56]
Whereas crystallization and melting are first-order phase transitions, the glass transition is non.[57] The glass transition shares features of second-order phase transitions (such as discontinuity in the heat capacity, as shown in the figure), just it is more often than not not considered a thermodynamic transition between equilibrium states.
Mixing behavior

Phase diagram of the typical mixing behavior of weakly interacting polymer solutions, showing spinodal curves and binodal coexistence curves.
In general, polymeric mixtures are far less miscible than mixtures of minor molecule materials. This consequence results from the fact that the driving forcefulness for mixing is unremarkably entropy, not interaction free energy. In other words, miscible materials commonly form a solution not because their interaction with each other is more favorable than their self-interaction, but because of an increase in entropy and hence gratuitous energy associated with increasing the corporeality of book bachelor to each component. This increase in entropy scales with the number of particles (or moles) beingness mixed. Since polymeric molecules are much larger and hence generally accept much college specific volumes than small-scale molecules, the number of molecules involved in a polymeric mixture is far smaller than the number in a small molecule mixture of equal volume. The energetics of mixing, on the other hand, is comparable on a per volume basis for polymeric and small-scale molecule mixtures. This tends to increase the free energy of mixing for polymer solutions and thereby making solvation less favorable, and thereby making the availability of concentrated solutions of polymers far rarer than those of modest molecules.
Furthermore, the phase behavior of polymer solutions and mixtures is more complex than that of small molecule mixtures. Whereas most modest molecule solutions showroom only an upper critical solution temperature stage transition (UCST), at which phase separation occurs with cooling, polymer mixtures commonly showroom a lower critical solution temperature phase transition (LCST), at which phase separation occurs with heating.
In dilute solutions, the properties of the polymer are characterized by the interaction between the solvent and the polymer. In a good solvent, the polymer appears swollen and occupies a large volume. In this scenario, intermolecular forces betwixt the solvent and monomer subunits dominate over intramolecular interactions. In a bad solvent or poor solvent, intramolecular forces dominate and the chain contracts. In the theta solvent, or the state of the polymer solution where the value of the second virial coefficient becomes 0, the intermolecular polymer-solvent repulsion balances exactly the intramolecular monomer-monomer allure. Nether the theta condition (also chosen the Flory condition), the polymer behaves like an ideal random gyre. The transition between the states is known as a coil–globule transition.
Inclusion of plasticizers
Inclusion of plasticizers tends to lower Tg and increase polymer flexibility. Addition of the plasticizer volition also modify dependence of the glass-transition temperature Tm on the cooling rate.[58] The mobility of the chain can further change if the molecules of plasticizer give rise to hydrogen bonding formation. Plasticizers are generally small molecules that are chemically similar to the polymer and create gaps between polymer chains for greater mobility and fewer interchain interactions. A skilful example of the activity of plasticizers is related to polyvinylchlorides or PVCs. A uPVC, or unplasticized polyvinylchloride, is used for things such as pipes. A pipe has no plasticizers in it, because information technology needs to remain stiff and heat-resistant. Plasticized PVC is used in wearable for a flexible quality. Plasticizers are as well put in some types of cling picture to brand the polymer more flexible.
Chemic backdrop
The attractive forces between polymer bondage play a large part in determining the polymer'due south backdrop. Because polymer chains are so long, they have many such interchain interactions per molecule, amplifying the outcome of these interactions on the polymer properties in comparison to attractions between conventional molecules. Different side groups on the polymer can lend the polymer to ionic bonding or hydrogen bonding between its own bondage. These stronger forces typically result in higher tensile strength and higher crystalline melting points.
The intermolecular forces in polymers tin be affected by dipoles in the monomer units. Polymers containing amide or carbonyl groups tin class hydrogen bonds betwixt adjacent chains; the partially positively charged hydrogen atoms in N-H groups of one chain are strongly attracted to the partially negatively charged oxygen atoms in C=O groups on another. These strong hydrogen bonds, for example, result in the high tensile strength and melting bespeak of polymers containing urethane or urea linkages. Polyesters have dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogen atoms in H-C groups. Dipole bonding is not as strong as hydrogen bonding, and then a polyester'due south melting indicate and strength are lower than Kevlar's (Twaron), but polyesters have greater flexibility. Polymers with non-polar units such as polyethylene interact only through weak Van der Waals forces. As a result, they typically accept lower melting temperatures than other polymers.
When a polymer is dispersed or dissolved in a liquid, such every bit in commercial products like paints and glues, the chemic properties and molecular interactions influence how the solution flows and can fifty-fifty lead to self-associates of the polymer into circuitous structures. When a polymer is applied every bit a coating, the chemical properties will influence the adhesion of the coating and how it interacts with external materials, such as superhydrophobic polymer coatings leading to water resistance. Overall the chemical properties of a polymer are of import elements for designing new polymeric material products.
Optical backdrop
Polymers such as PMMA and HEMA:MMA are used as matrices in the gain medium of solid-land dye lasers, as well known as solid-state dye-doped polymer lasers. These polymers have a high surface quality and are also highly transparent so that the laser properties are dominated by the laser dye used to dope the polymer matrix. These blazon of lasers, that too belong to the course of organic lasers, are known to yield very narrow linewidths which is useful for spectroscopy and analytical applications.[59] An important optical parameter in the polymer used in laser applications is the change in refractive index with temperature also known every bit dn/dT. For the polymers mentioned hither the (dn/dT) ~ −1.four × 10−4 in units of K−1 in the 297 ≤ T ≤ 337 G range.[60]
Electrical properties
Most conventional polymers such as polyethylene are electric insulators, but the development of polymers containing π-conjugated bonds has led to a wealth of polymer-based semiconductors, such as polythiophenes. This has led to many applications in the field of organic electronics.
Applications
Present, constructed polymers are used in most all walks of life. Modern lodge would await very unlike without them. The spreading of polymer use is connected to their unique properties: low density, low cost, good thermal/electrical insulation properties, high resistance to corrosion, low-energy demanding polymer manufacture and facile processing into final products. For a given application, the properties of a polymer tin can be tuned or enhanced by combination with other materials, every bit in composites. Their application allows to save energy (lighter cars and planes, thermally insulated buildings), protect food and drinking water (packaging), save land and lower utilise of fertilizers (synthetic fibres), preserve other materials (coatings), protect and salve lifes (hygiene, medical applications). A representative, non-exhaustive list of applications is given below.
- Wearable, sportswear and accessories: polyester and PVC clothing, spandex, sport shoes, wetsuits, footballs and billiard assurance, skis and snowboards, rackets, parachutes, sails, tents and shelters.
- Electronic and photonic technologies: organic field outcome transistors (OFET), light emitting diodes (OLED) and solar cells, television components, compact discs (CD), photoresists, holography.
- Packaging and containers: films, bottles, food packaging, barrels.
- Insulation: electrical and thermal insulation, spray foams.
- Construction and structural applications: garden furniture, PVC windows, flooring, sealing, pipes.
- Paints, glues and lubricants: varnish, adhesives, dispersants, anti-graffiti coatings, antifouling coatings, non-stick surfaces, lubricants.
- Car parts: tires, bumpers, windshields, windscreen wipers, fuel tanks, automobile seats.
- Household items: buckets, kitchenware, toys (e.g., construction sets and Rubik's cube).
- Medical applications: blood bag, syringes, rubber gloves, surgical suture, contact lenses, prosthesis, controlled drug delivery and release, matrices for prison cell growth.
- Personal hygiene and healthcare: diapers using superabsorbent polymers, toothbrushes, cosmetics, shampoo, condoms.
- Security: personal protective equipment, bulletproof vests, space suits, ropes.
- Separation technologies: constructed membranes, fuel prison cell membranes, filtration, ion-exchange resins.
- Money: polymer banknotes and payment cards.
- 3D press.
Standardized classification
There are multiple conventions for naming polymer substances. Many unremarkably used polymers, such as those found in consumer products, are referred to by a common or niggling name. The trivial name is assigned based on historical precedent or popular usage rather than a standardized naming convention. Both the American Chemic Society (ACS)[61] and IUPAC[62] have proposed standardized naming conventions; the ACS and IUPAC conventions are similar just not identical.[63] Examples of the differences betwixt the various naming conventions are given in the table below:
| Mutual name | ACS name | IUPAC proper name |
|---|---|---|
| Poly(ethylene oxide) or PEO | Poly(oxyethylene) | Poly(oxyethylene) |
| Poly(ethylene terephthalate) or PET | Poly(oxy-ane,2-ethanediyloxycarbonyl-1,four-phenylenecarbonyl) | Poly(oxyethyleneoxyterephthaloyl) |
| Nylon 6 or Polyamide 6 | Poly[imino(ane-oxo-one,half-dozen-hexanediyl)] | Poly[azanediyl(one-oxohexane-one,6-diyl)] |
In both standardized conventions, the polymers' names are intended to reflect the monomer(s) from which they are synthesized (source based classification) rather than the precise nature of the repeating subunit. For example, the polymer synthesized from the unproblematic alkene ethene is called polyethene, retaining the -ene suffix even though the double bail is removed during the polymerization process:
-
 →
→
- However, IUPAC structure based classification is based on naming of the preferred constitutional repeating unit.[64]
Characterization
Polymer characterization spans many techniques for determining the chemical composition, molecular weight distribution, and physical properties. Select common techniques include the following:
- Size-exclusion chromatography (likewise called gel permeation chromatography), sometimes coupled with static light scattering, can used to decide the number-average molecular weight, weight-average molecular weight, and dispersity.
- Scattering techniques, such equally static calorie-free handful and modest-angle neutron scattering, are used to determine the dimensions (radius of gyration) of macromolecules in solution or in the melt. These techniques are besides used to characterize the 3-dimensional structure of microphase-separated block polymers, polymeric micelles, and other materials.
- Wide-bending X-ray scattering (also called wide-bending X-ray diffraction) is used to determine the crystalline structure of polymers (or lack thereof).
- Spectroscopy techniques, including Fourier-transform infrared spectroscopy, Raman spectroscopy, and nuclear magnetic resonance spectroscopy, tin be used to determine the chemical composition.
- Differential scanning calorimetry is used to characterize the thermal properties of polymers, such equally the glass-transition temperature, crystallization temperature, and melting temperature. The glass-transition temperature can also be determined by dynamic mechanical analysis.
- Thermogravimetry is a useful technique to evaluate the thermal stability of the polymer.
- Rheology is used to narrate the flow and deformation behavior. It can be used to decide the viscosity, modulus, and other rheological properties. Rheology is also often used to decide the molecular architecture (molecular weight, molecular weight distribution, branching) and to understand how the polymer can exist candy.
Degradation

A plastic detail with thirty years of exposure to heat and cold, brake fluid, and sunlight. Notice the discoloration, swelling, and crazing of the material
Polymer deposition is a modify in the properties—tensile forcefulness, color, shape, or molecular weight—of a polymer or polymer-based production under the influence of one or more environmental factors, such as estrus, light, and the presence of certain chemicals, oxygen, and enzymes. This alter in properties is oft the outcome of bond breaking in the polymer backbone (chain scission) which may occur at the concatenation ends or at random positions in the chain.
Although such changes are frequently undesirable, in some cases, such as biodegradation and recycling, they may be intended to prevent environmental pollution. Degradation can too exist useful in biomedical settings. For instance, a copolymer of polylactic acrid and polyglycolic acrid is employed in hydrolysable stitches that slowly dethrone afterward they are applied to a wound.
The susceptibility of a polymer to degradation depends on its structure. Epoxies and chains containing aromatic functionalities are especially susceptible to UV degradation while polyesters are susceptible to deposition by hydrolysis. Polymers containing an unsaturated backbone degrade via ozone cracking. Carbon based polymers are more susceptible to thermal degradation than inorganic polymers such as polydimethylsiloxane and are therefore not ideal for well-nigh high-temperature applications.
The degradation of polyethylene occurs by random scission—a random breakage of the bonds that concur the atoms of the polymer together. When heated above 450 °C, polyethylene degrades to course a mixture of hydrocarbons. In the instance of chain-cease scission, monomers are released and this procedure is referred to as unzipping or depolymerization. Which machinery dominates will depend on the type of polymer and temperature; in general, polymers with no or a single small-scale substituent in the repeat unit will decompose via random-chain scission.
The sorting of polymer waste material for recycling purposes may be facilitated past the apply of the resin identification codes developed by the Guild of the Plastics Industry to identify the type of plastic.
Product failure

Chlorine attack of acetal resin plumbing joint
Failure of prophylactic-critical polymer components can cause serious accidents, such as fire in the case of cracked and degraded polymer fuel lines. Chlorine-induced cracking of acetal resin plumbing joints and polybutylene pipes has caused many serious floods in domestic properties, especially in the U.s.a. in the 1990s. Traces of chlorine in the h2o supply attacked polymers present in the plumbing, a problem which occurs faster if any of the parts take been poorly extruded or injection molded. Attack of the acetal articulation occurred because of faulty molding, leading to cracking along the threads of the fitting where in that location is stress concentration.

Ozone-induced groovy in natural rubber tubing
Polymer oxidation has acquired accidents involving medical devices. One of the oldest known failure modes is ozone great acquired past chain scission when ozone gas attacks susceptible elastomers, such as natural rubber and nitrile safe. They possess double bonds in their repeat units which are broken during ozonolysis. Cracks in fuel lines can penetrate the bore of the tube and cause fuel leakage. If neat occurs in the engine compartment, electrical sparks can ignite the gasoline and can cause a serious burn down. In medical use degradation of polymers tin atomic number 82 to changes of concrete and chemic characteristics of implantable devices.[65]
Nylon 66 is susceptible to acid hydrolysis, and in i accident, a fractured fuel line led to a spillage of diesel into the road. If diesel fuel leaks onto the road, accidents to following cars can be caused by the slippery nature of the eolith, which is like black ice. Furthermore, the asphalt concrete road surface volition suffer harm as a result of the diesel fuel dissolving the asphaltenes from the composite material, this resulting in the deposition of the asphalt surface and structural integrity of the route.
Come across also
| | Wikimedia Commons has media related to Polymers. |
- Biopolymer
- Ideal chain
- Catenation
- Inorganic polymer
- Important publications in polymer chemistry
- Oligomer
- Polymer adsorption
- Polymer classes
- Polymer engineering
- Polymerization
- Polymery (botany)
- Reactive compatibilization
- Sequence-controlled polymer
- Shape-memory polymer
- Sol–gel process
- Supramolecular polymer
- Thermoplastic
- Thermosetting polymer
References
- ^ Roiter, Y.; Minko, S. (2005). "AFM Unmarried Molecule Experiments at the Solid-Liquid Interface: In Situ Conformation of Adsorbed Flexible Polyelectrolyte Chains". Journal of the American Chemical Society. 127 (45): 15688–15689. doi:ten.1021/ja0558239. PMID 16277495.
- ^ IUPAC, Compendium of Chemical Terminology, second ed. (the "Gilded Volume") (1997). Online corrected version: (2006–) "polymer". doi:10.1351/goldbook.P04735
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gilt Book") (1997). Online corrected version: (2006–) "macromolecule (polymer molecule)". doi:10.1351/goldbook.M03667
- ^ "Polymer – Definition of polymer". The Free Dictionary. Retrieved 23 July 2013.
- ^ "Define polymer". Dictionary Reference . Retrieved 23 July 2013.
- ^ "Polymer on Britannica".
- ^ Painter, Paul C.; Coleman, Michael Grand. (1997). Fundamentals of polymer scientific discipline: an introductory text. Lancaster, Pa.: Technomic Pub. Co. p. 1. ISBN978-1-56676-559-6.
- ^ McCrum, N. G.; Buckley, C. P.; Bucknall, C. B. (1997). Principles of polymer engineering. Oxford; New York: Oxford University Printing. p. 1. ISBN978-0-19-856526-0.
- ^ If two substances had molecular formulae such that 1 was an integer multiple of the other – e.g., acetylene (C2H2) and benzene (C6H6) – Berzelius chosen the multiple formula "polymeric". Run into: Jöns Jakob Berzelius (1833) "Isomerie, Unterscheidung von damit analogen Verhältnissen" (Isomeric, stardom from relations analogous to information technology), Jahres-Bericht über die Fortschitte der physischen Wissenschaften …, 12: 63–67. From page 64: "Um diese Art von Gleichheit in der Zusammensetzung, bei Ungleichheit in den Eigenschaften, bezeichnen zu können, möchte ich für diese Körper dice Benennung polymerische (von πολυς mehrere) vorschlagen." (In club to be able to denote this type of similarity in limerick [which is accompanied] past differences in properties, I would similar to propose the designation "polymeric" (from πολυς, several) for these substances.)
Originally published in 1832 in Swedish every bit: Jöns Jacob Berzelius (1832) "Isomeri, dess distinktion från dermed analoga förhållanden," Årsberättelse om Framstegen i Fysik och Kemi, pages 65–70; the word "polymeriska" appears on folio 66. - ^ Jensen, William B. (2008). "Inquire the Historian: The origin of the polymer concept" (PDF). Periodical of Chemical Education. 85 (5): 624–625. Bibcode:2008JChEd..85..624J. doi:x.1021/ed085p624. Archived from the original (PDF) on 2018-06-xviii. Retrieved 2013-03-04 .
- ^ Staudinger, H (1920). "Über Polymerisation" [On polymerization]. Berichte der Deutschen Chemischen Gesellschaft (in High german). 53 (half dozen): 1073–1085. doi:10.1002/cber.19200530627.
- ^ Allcock, Harry R.; Lampe, Frederick W.; Mark, James E. (2003). Contemporary Polymer Chemical science (3 ed.). Pearson Education. p. 21. ISBN978-0-13-065056-6.
- ^ "World Plastics Product" (PDF).
- ^ Hurley, Paul East. (May 1981). "History of Natural Rubber". Periodical of Macromolecular Scientific discipline: Part A - Chemistry. 15 (7): 1279–1287. doi:x.1080/00222338108056785. ISSN 0022-233X.
- ^ a b Feldman, Dorel (Jan 2008). "Polymer History". Designed Monomers and Polymers. eleven (1): 1–fifteen. doi:10.1163/156855508X292383. ISSN 1568-5551. S2CID 219539020.
- ^ Staudinger, H. (1920-06-12). "Über Polymerisation". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 53 (six): 1073–1085. doi:10.1002/cber.19200530627. ISSN 0365-9488.
- ^ "The Nobel Prize in Chemical science 1953". NobelPrize.org . Retrieved 2020-06-25 .
- ^ Feldman, Dorel (2008-01-01). "Polymer History". Designed Monomers and Polymers. 11 (1): 1–15. doi:10.1163/156855508X292383. S2CID 219539020.
- ^ "Lord Todd: the state of chemistry". Chemical & Applied science News Archive. 58 (40): 28–33. 1980-ten-06. doi:x.1021/cen-v058n040.p028. ISSN 0009-2347.
- ^ Sperling, Fifty. H. (Leslie Howard) (2006). Introduction to physical polymer science. Hoboken, N.J.: Wiley. p. 10. ISBN978-0-471-70606-9.
- ^ Sperling, p. xi
- ^ Sperling, p. 15
- ^ Sperling, p. 29
- ^ Bower, David I. (2002). An introduction to polymer physics. Cambridge University Press. ISBN9780511801280.
- ^ Rudin, p.17
- ^ Cowie, p.4
- ^ Sperling, p. xxx
- ^ a b Rubinstein, Michael; Colby, Ralph H. (2003). Polymer physics . Oxford; New York: Oxford University Press. p. 6. ISBN978-0-xix-852059-seven.
- ^ McCrum, p. 30
- ^ Rubinstein, p. 3
- ^ McCrum, p. 33
- ^ Rubinstein, pp. 23–24
- ^ Painter, p. 22
- ^ De Gennes, Pierre Gilles (1979). Scaling concepts in polymer physics. Ithaca, N.Y.: Cornell University Press. ISBN978-0-8014-1203-5.
- ^ Rubinstein, p. 5
- ^ McCrum, p. 37
- ^ Introduction to Polymer Science and Chemical science: A Problem-Solving Approach By Manas Chanda
- ^ O'Driscoll, Grand.; Amin Sanayei, R. (July 1991). "Chain-length dependence of the glass transition temperature". Macromolecules. 24 (fifteen): 4479–4480. Bibcode:1991MaMol..24.4479O. doi:10.1021/ma00015a038.
- ^ Pokrovskii, V. Northward. (2010). The Mesoscopic Theory of Polymer Dynamics. Springer Serial in Chemical Physics. Vol. 95. Bibcode:2010mtpd.book.....P. doi:x.1007/978-90-481-2231-viii. ISBN978-90-481-2230-ane.
- ^ Edwards, South. F. (1967). "The statistical mechanics of polymerized fabric" (PDF). Proceedings of the Concrete Gild. 92 (1): 9–xvi. Bibcode:1967PPS....92....9E. doi:10.1088/0370-1328/92/i/303. [ permanent expressionless link ]
- ^ Painter, p. 14
- ^ a b c d Rudin p.18-20
- ^ a b c Cowie p.104
- ^ "Periodic copolymer". IUPAC Compendium of Chemical Terminology, 2nd ed. (the "Aureate Volume"). International Matrimony of Pure and Applied Chemistry. 2014. doi:10.1351/goldbook.P04494. Retrieved 9 April 2020.
- ^ Painter, p. 15
- ^ Sperling, p. 47
- ^ Lutz, Jean-François; Ouchi, Makoto; Liu, David R.; Sawamoto, Mitsuo (2013-08-09). "Sequence-Controlled Polymers". Science. 341 (6146): 1238149. doi:10.1126/science.1238149. ISSN 0036-8075. PMID 23929982. S2CID 206549042.
- ^ a b Bernd Tieke: Makromolekulare Chemie. three. Auflage, Wiley-VCH, Weinheim 2014, S. 295f (in German language).
- ^ a b Wolfgang Kaiser: Kunststoffchemie für Ingenieure. 3. Auflage, Carl Hanser, München 2011, Due south. 84.
- ^ Sayed, Abu (August 2014). "Types of polymer: Requirements of fibre forming polymer". Textile Apex.
- ^ Allcock, Harry R.; Lampe, Frederick W.; Mark, James Due east. (2003). Contemporary Polymer Chemistry (three ed.). Pearson Education. p. 546. ISBN978-0-13-065056-vi.
- ^ Rubinstein, p. 13
- ^ Ashby, Michael; Jones, David (1996). Applied science Materials (2 ed.). Butterworth-Heinermann. pp. 191–195. ISBN978-0-7506-2766-5.
- ^ Meyers, M. A.; Chawla, One thousand. K. (1999). Mechanical Beliefs of Materials. Cambridge Academy Press. p. 41. ISBN978-0-521-86675-0. Archived from the original on 2013-11-02. Retrieved 2018-12-31 .
- ^ Fried, Joel R. (2003). Polymer Scientific discipline & Applied science (second ed.). Prentice Hall. pp. 155–half-dozen. ISBN0-thirteen-018168-four.
- ^ Brandrup, J.; Immergut, Eastward.H.; Grulke, E.A. (1999). Polymer Handbook (iv ed.). Wiley-Interscience. ISBN978-0-471-47936-ix.
- ^ Meille, S.; Allegra, G.; Geil, P.; et al. (2011). "Definitions of terms relating to crystalline polymers (IUPAC Recommendations 2011)" (PDF). Pure and Applied Chemistry. 83 (ten): 1831–1871. doi:10.1351/PAC-REC-x-xi-xiii. S2CID 98823962. Retrieved 2018-12-31 .
- ^ Capponi, S.; Alvarez, F.; Racko, D. (2020), "Free Volume in a PVME Polymer–Water Solution", Macromolecules, Xxx (XXX): Thirty–XXX, Bibcode:2020MaMol..53.4770C, doi:ten.1021/acs.macromol.0c00472, hdl:10261/218380, S2CID 219911779
- ^ Duarte, F. J. (1999). "Multiple-prism grating solid-state dye light amplification by stimulated emission of radiation oscillator: optimized architecture". Practical Eyes. 38 (xxx): 6347–6349. Bibcode:1999ApOpt..38.6347D. doi:10.1364/AO.38.006347. PMID 18324163.
- ^ Duarte, F. J. (2003). Tunable Light amplification by stimulated emission of radiation Eyes. New York: Elsevier Academic. ISBN978-0122226960.
- ^ CAS: Index Guide, Appendix 4 ((c) 1998)
- ^ IUPAC (1976). "Nomenclature of Regular Single-Strand Organic Polymers". Pure Appl. Chem. 48 (3): 373–385. doi:x.1351/pac197648030373.
- ^ Wilks, East.S. "Macromolecular Classification Annotation No. 18". Archived from the original on 25 September 2003.
- ^ Hiorns, R. C.; Boucher, R. J.; Duhlev, R.; Hellwich, Karl-Heinz; Hodge, Philip; Jenkins, Aubrey D.; Jones, Richard Thousand.; Kahovec, Jaroslav; Moad, Graeme; Ober, C. K.; Smith, D. West. (2012-10-03). "A cursory guide to polymer classification (IUPAC Technical Report)". Pure and Practical Chemistry. 84 (10): 2167–2169. doi:10.1351/PAC-REP-12-03-05. ISSN 0033-4545. S2CID 95629051.
- ^ Iakovlev, V.; Guelcher, S.; Bendavid, R. (Baronial 28, 2015). "Degradation of polypropylene in vivo: A microscopic analysis of meshes explanted from patients". Journal of Biomedical Materials Research Part B: Practical Biomaterials. 105 (2): 237–248. doi:x.1002/jbm.b.33502. PMID 26315946.
Bibliography
- Cowie, J. M. Grand. (John McKenzie Grant) (1991). Polymers: chemical science and physics of modern materials . Glasgow: Blackie. ISBN978-0-412-03121-2.
- Hall, Christopher (1989). Polymer materials (second ed.). London; New York: Macmillan. ISBN978-0-333-46379-6.
- Rudin, Alfred (1982). The elements of polymer science and engineering . Academic Printing. ISBN978-0-12-601680-ii.
- Wright, David C. (2001). Environmental Stress Cracking of Plastics. RAPRA. ISBN978-1-85957-064-7.
External links
| | Look up polymer in Wiktionary, the free dictionary. |
- How to Analyze Polymers Using X-ray Diffraction
- Polymer Chemical science Hypertext, Educational resource
- The Macrogalleria
- Introduction to Polymers
- Glossary of Polymer Abbreviations
ericksonfrect1987.blogspot.com
Source: https://en.wikipedia.org/wiki/Polymer




Belum ada Komentar untuk "what is a branched polymer? cite your source and be sure to define this term in your own words."
Posting Komentar